
In the form of heat this energy is reduced to a state of maximumdisorder in which each individual movement is neutralized by statisticallaws. Heat is energy it is kinetic energy that resultsfrom the movement of molecules in a gas or the vibration of atoms in asolid. There seems to be a contradiction between the first and second principles.One says that heat and energy are two dimensions of the same nature theother says they are not, since potential energy is degraded irreversiblyto an inferior, less noble, lower-quality form-heat. But how can degraded energy, or its hierarchy,or the process of degradation be truly represented?

Yet some scientists consider it intuitively theyneed only refer mentally to actual states such as disorder, waste, andthe loss of time or information. The concept of entropy is particularly abstract and by the same tokendifficult to present.

The irreversible increase of this nondisposable energy in the universeis measured by the abstract dimension that Clausius in 1865 called entropy(from the Greek entrope, change). This does not mean thatthe energy is destroyed it means that it becomes unavailable for producingwork.
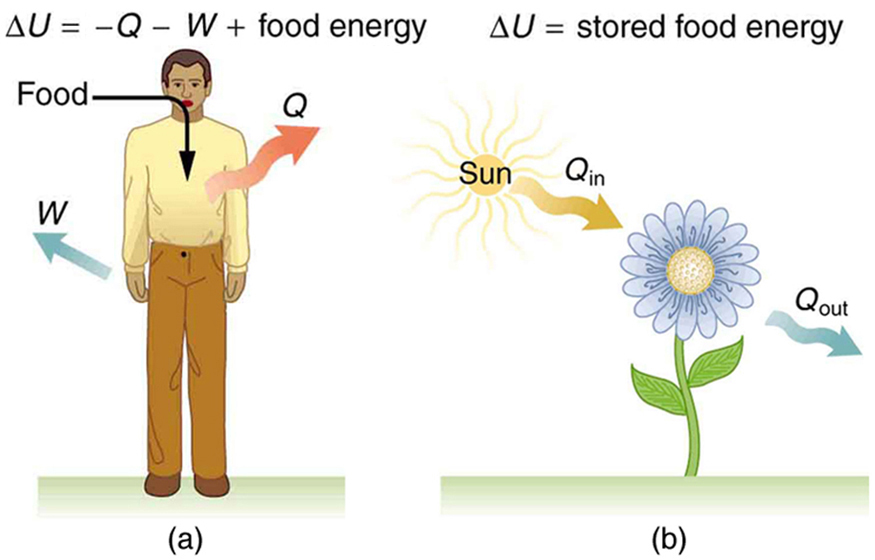
But the reverse (heat into physical energy, for example)cannot be fully accomplished without outside help or without an inevitableloss of energy in the form of irretrievable heat. In fact physical, chemical, and electrical energy can be completelychanged into heat. This first principle considers heat and energy as twomagnitudes of the same physical natureĪbout 1850 the studies of Lord Kelvin, Carnot, and Clausius of the exchangesof energy in thermal machines revealed that there is a hierarchy amongthe various forms of energy and an imbalance in their transformations.This hierarchy and this imbalance are the basis of the formulation of thesecond principle. The first principle establishes the equivalence of the different formsof energy (radiant, chemical, physical, electrical, and thermal), the possibilityof transformation from one form to another, and the laws that govern thesetransformations. This is the principleof the degradation of energy. The second law of thermodynamics states that the quality of this energy is degraded irreversibly. This is the principle of theconservation of energy. The first law of thermodynamics says that the total quantityof energy in the universe remains constant. The universe in its totality might be considered a closedsystem of this type this would allow the two laws to be applied to it.


 0 kommentar(er)
0 kommentar(er)
